Why Is A Platinum Wire Used For Flame Test. Why Not Iron Wire Nor Aluminium Wire.
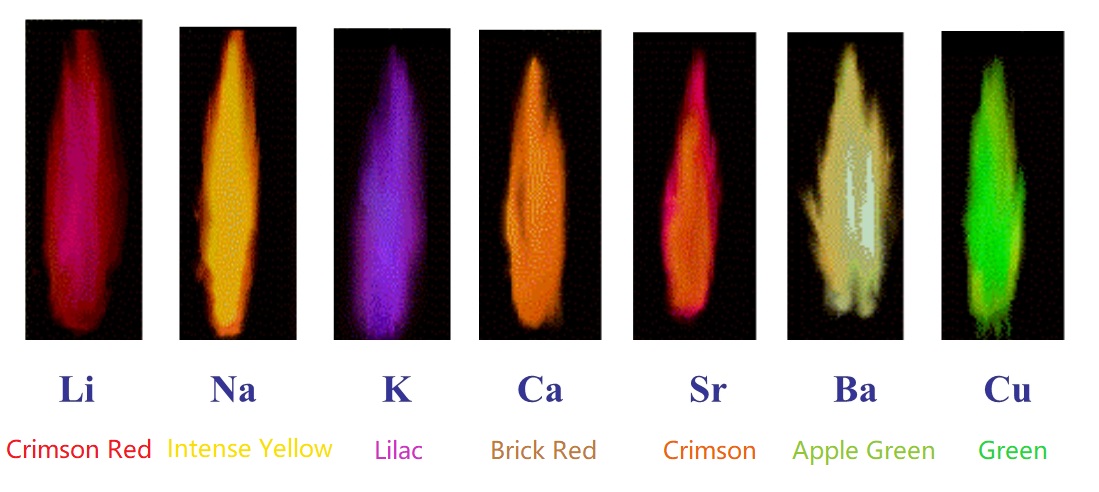
Flame test is based on the rule that each element's characteristic emission spectrum, the color of flames in general also depends on temperature and ionic valency. Flame test a simple and convenient method helps us to detect the presence of certain elements (mainly for alkali metal). Following is the frame colors of some common metals.
Because of the unique characters of platinum as follows,
Lithium: Crimson Red Sodium: Intense Yellow PotassiumLilac Calcium: Brick Red Strontium: Crimson Barium: Apple Green Copper: Green

Generally speaking, we are told to use a platinum wire dip into the powder or solution to be tested, and then place the tip into the hottest portion of a flame, then we could see the flame color of the element insida the powder/solution.
Why is a platinum wire used for flame test? Why not iron wire nor aluminium wire?
Because of the unique characters of platinum as follows,
1, Platinum is chemically inert, meaning that it is not oxidised under the high temperature of the flame from a bunsen burner, even at high temperatures, it remains unattacked by free radicals / acid radicals. (clean by hydrochloric acid or nitric acid after use)
2, Platinum doesn't impart any color to the flame, it ensures that we only see the color of element what we are burning.
3, The melting point of platinum is very high at 2041ºC. Glass rod softens and melts at a temperatures around 500ºC, can't sustain long under the flame of a bunsen burner and alcohol blast burner, even a common glass alcohol burner can reach 500ºC.
Other metal materials, such as iron, copper, aluminium, nickel will bring in other color when doing a flame test. Other inorganic materials, such as glass and porcelain, can not sustain at high temperatures. (or oxidized, or bring in other color)
 Espanol
Espanol English
English